Kurtis MSD Meconium Suction Device – Neonatal Airway Management
How fast will this ship?
How fast will this ship?
Typically ships in 0-2 days
What does this mean?
Health Canada License
Health Canada License
- Manufaturer:
- Device Class:
- MDL: 23159
Return Policy
Return Policy
30 DAYS
Return Request Form
*All returns must be unopened/unused
Description
Description
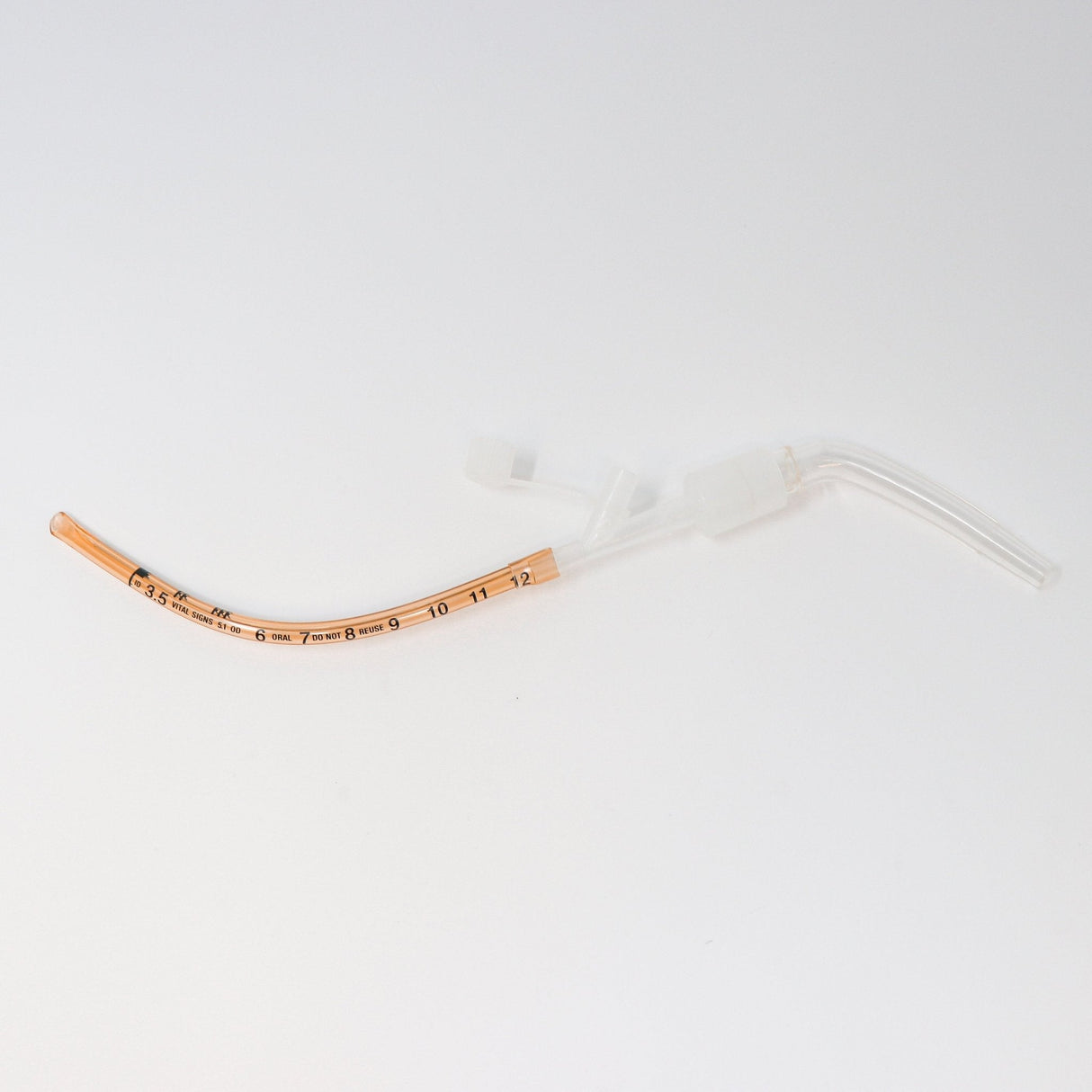
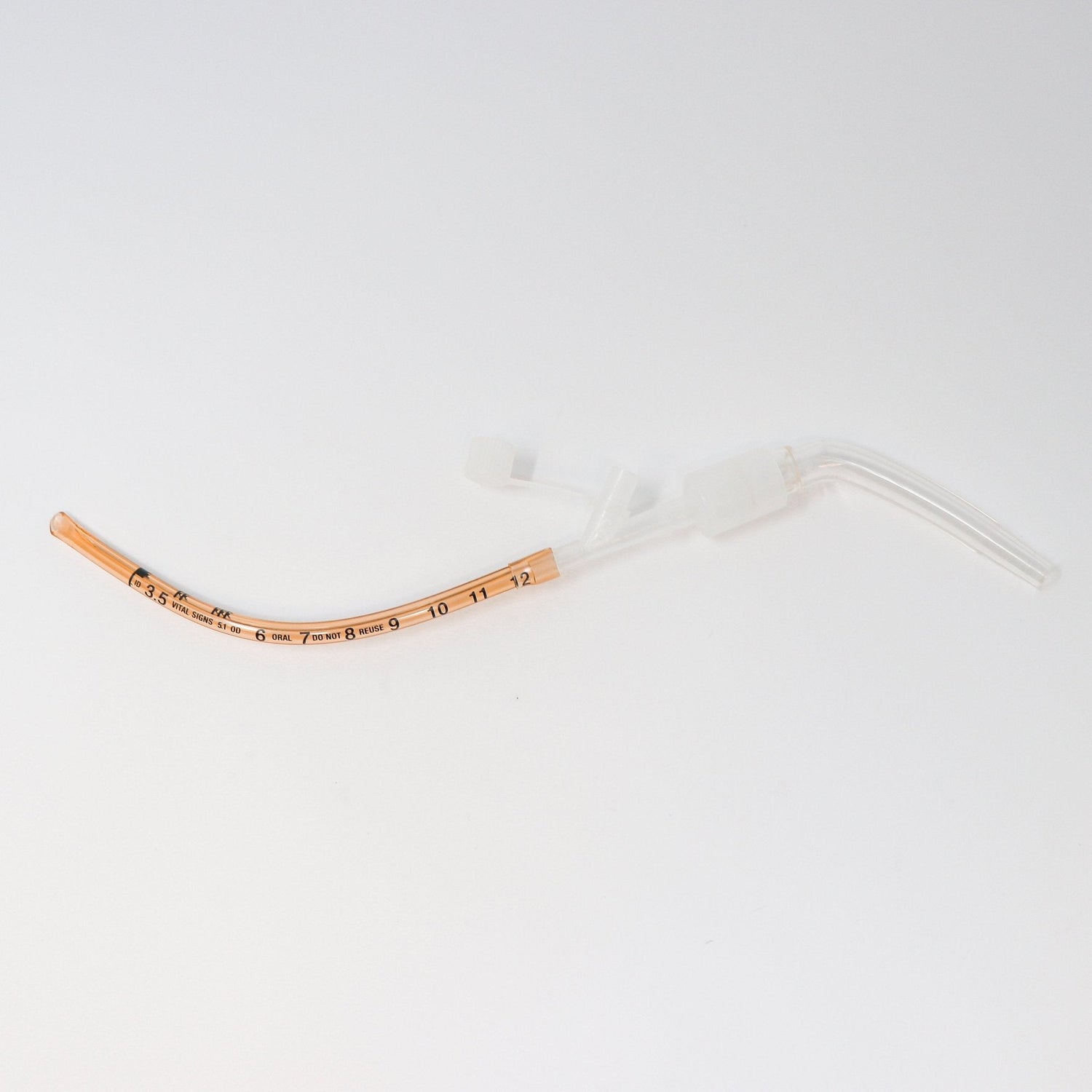
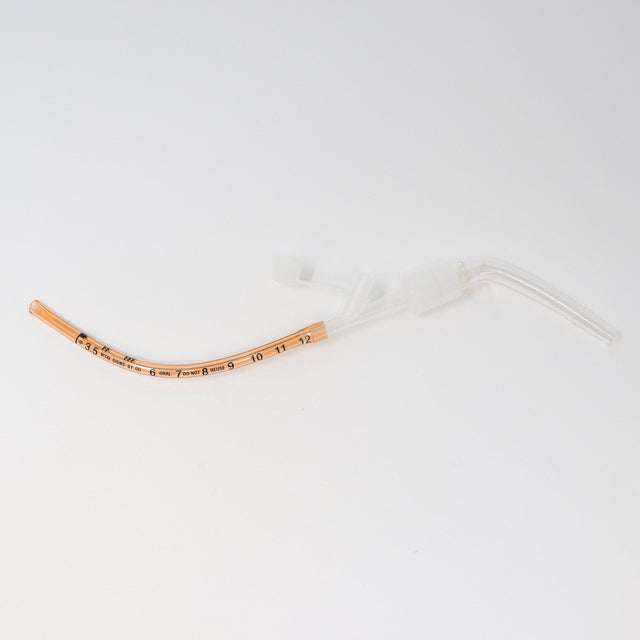
The Kurtis MSD Meconium Suction Device (MSD) was designed by a neonatologist to streamline neonatal resuscitation when every second matters. By combining multiple airway management functions into a single, preassembled device, the MSD reduces setup time and simplifies the critical process of meconium suctioning.
Its wire-reinforced, malleable tracheal tube eliminates the need for a stylet, while the low deadspace adapter allows direct attachment of a resuscitator for positive pressure ventilation if required. Suction tubing can be connected before intubation, ensuring immediate readiness.
Uniquely, the MSD functions both as a suction catheter and artificial airway, enabling rapid intervention by a single provider and providing continuity during transport. This innovative dual functionality improves provider efficiency, patient safety, and neonatal outcomes.
Key Features
-
Integrates tracheal tube and suction adapter into one device
-
Preassembled and instantly ready to use
-
Allows suction tubing connection before intubation
-
Wire-reinforced, malleable tracheal tube removes need for stylet
-
Low deadspace adapter for direct resuscitator attachment
-
Operated by a single provider for rapid, safe intervention
-
Functions as both a suction catheter and artificial airway during transport
Payment & Security
Payment methods
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.